FDA Greenlights Lenacapavir Based on Utah Biochemist’s Work
The U.S. Food and Drug Administration (FDA) has officially approved lenacapavir, a groundbreaking drug for HIV Drug prevention, rooted in decades of research by University of Utah biochemist Wesley Sundquist. This medication, marketed under the name Yeztugo, was developed by Gilead Sciences and is already being hailed as a game-changer in the fight against HIV.
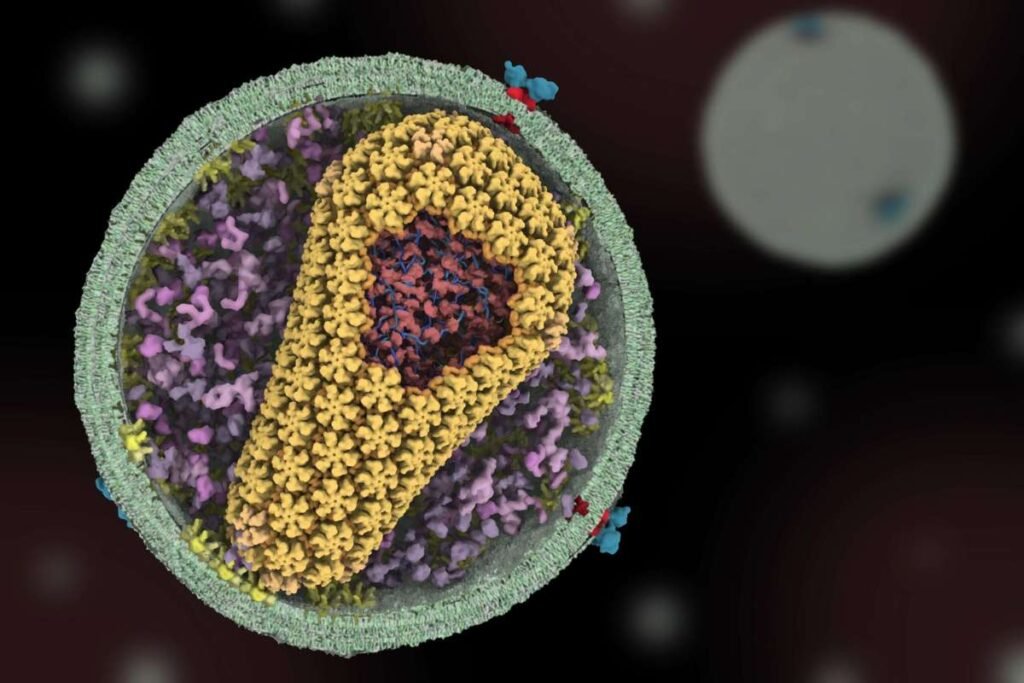
Lenacapavir is designed to HIV Drug by targeting the virus’s protein shell—known as the capsid—essentially halting its ability to replicate. Sundquist’s laboratory laid the foundational understanding of this protein structure back in the late 1990s. His team discovered how sensitive the virus’s shell is to small changes, revealing a new path for treatment. Gilead Sciences took note of these findings and eventually collaborated with Sundquist to create lenacapavir.
Unlike many existing HIV medications, lenacapavir stands out for its long-lasting protection. Administered just twice a year, it offers a six-month shield against the virus. According to Sundquist, clinical trials have shown it to be “more potent than any drug available,” with remarkable efficacy and durability.
Clinical Trials Show Strong HIV Prevention
Lenacapavir’s effectiveness was demonstrated in major Phase 3 trials conducted in HIV-affected regions like South Africa and Uganda. These trials involved over 2,000 women, none of whom contracted HIV after receiving a dose of the HIV Drug . These results mark a major step forward in preventive medicine for populations at high risk of HIV infection.
Currently, about 40 million people worldwide are living with HIV, with roughly 600,000 deaths annually. In the U.S. alone, around 31,000 new infections are reported every year. Sundquist believes lenacapavir has the potential to shift these global numbers significantly. He emphasized, “This could change that trajectory. Of course, the rollout has to be funded and successful, but there’s no reason to think this won’t have a major impact.”
The approval by the FDA now makes the drug available for use in the United States, opening new possibilities for more effective and manageable HIV prevention strategies.
A Career of Impact and a Vision for the Future
Sundquist’s contributions to HIV research have earned him widespread recognition. Just last week, he received the prestigious Warren Alpert Prize from Harvard University, and in April, he was named among Time magazine’s 100 most influential people in the world. These honors highlight the global significance of his work.
Despite these accolades, Sundquist remains focused on what lies ahead. He stated that the ultimate goal—an HIV Drug vaccine—remains elusive. “We still need a vaccine. That would be even better because then you could give everyone the vaccine and protect everyone, not just at-risk individuals,” he said.
Reflecting on the role of science in medicine, Sundquist added, “We’re driven by curiosity to discover things that we don’t understand. The same thing that drives people to climb mountains drives us to discover how a molecular machine works.”
His commitment ensures that the fight against HIV Drug will continue, backed by scientific discovery and a vision for a healthier future.
Also Read :- Fact Check: Lenacapavir Prevents and Treats HIV but Does Not Cure the Virus