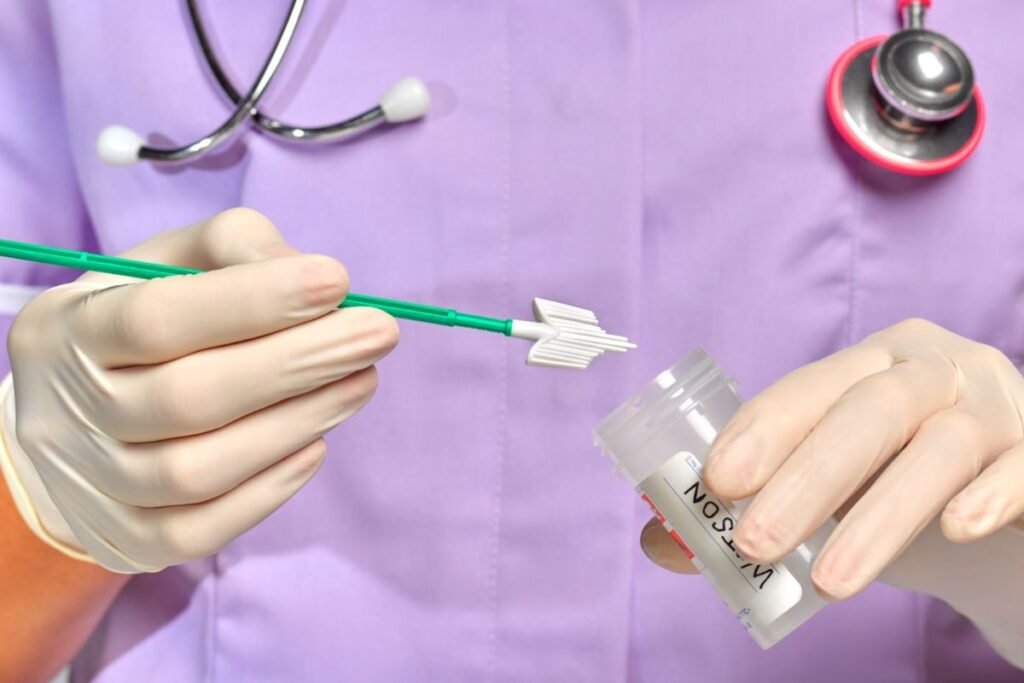
Federal health officials on Jan. 5 expanded cervical cancer screening guidelines, allowing women ages 30 to 65 to use self-administered at home HPV tests, aiming to improve screening rates by reducing discomfort and access barriers.
HRSA Expands Screening Options for Women 30 to 65
Federal guidelines are expanding cervical cancer screening beyond traditional Pap smears, allowing eligible women to choose at home HPV tests human papillomavirus tests, according to the Health Resources and Services Administration.
The updated recommendations, released Jan. 5, apply to women ages 30 to 65 at average risk of cervical cancer. They allow screening every five years using either a clinician-administered HPV test or an approved self-swab test.
Pap smears must still be offered, but they are no longer the preferred screening method for women 30 and older, the agency said. Women ages 21 to 29 should continue receiving Pap smears every three years.
The change reflects growing evidence that HPV testing is more effective at detecting cancer risk and that self-collection can remove barriers that keep many women from routine screening, according to HRSA and reporting by NBC News.
Studies Show Accuracy and Strong Patient Preference
At home HPV tests use a plastic wand, similar to a tampon, that is inserted into the vagina to collect cells for laboratory analysis. Pap smears require a speculum exam performed by a clinician, a process many women describe as painful or stressful.
Clinical studies show self-swab tests are comparable in accuracy to clinician-administered tests. In the SELF-CERV trial evaluating the Teal Wand, about 98% of participants successfully collected a valid sample.
Most participants reported the process was quick, with more than nine in ten completing the collection within five minutes. Nearly nine in ten said they would be more likely to stay up to date with screening if they could test at home.
“These tests meet women where they are,” the study authors reported, noting that comfort and convenience are key drivers of adherence.
Since 2024, the Food and Drug Administration has approved two self-swab HPV tests that can be ordered following a telehealth visit with a health care professional.
Experts Say Change Could Reverse Rising Cancer Rates
Cervical cancer is largely preventable, yet rates are rising among women in their 30s and early 40s, health officials said. Delayed screening and missed HPV vaccinations are cited as major contributors.
HPV vaccines prevent more than 90% of cervical cancers, and regular screening detects infections or precancerous changes early. Still, discomfort plays a significant role in screening delays. Studies show nearly one-third of women postpone exams due to pain or anxiety associated with speculum use.
Experts say self-collection could have the greatest impact in rural areas and underserved communities where access to clinics is limited. Screening participation could rise by as much as 20% across racial and ethnic groups, according to trial projections.
“Self-collection for high-risk HPV screening is an important and innovative breakthrough in the fight against cervical cancer,” experts wrote in a JAMA editorial on the updated guidelines. “By reducing testing barriers and expanding choice, these recommendations have the potential to increase screening rates and save lives.”
The American Cancer Society has also endorsed at-Home HPV Tests, although it continues to prefer clinician-administered testing every five years.
Health officials emphasized that women should discuss screening options with their providers to determine what is best for their individual risk and medical history.