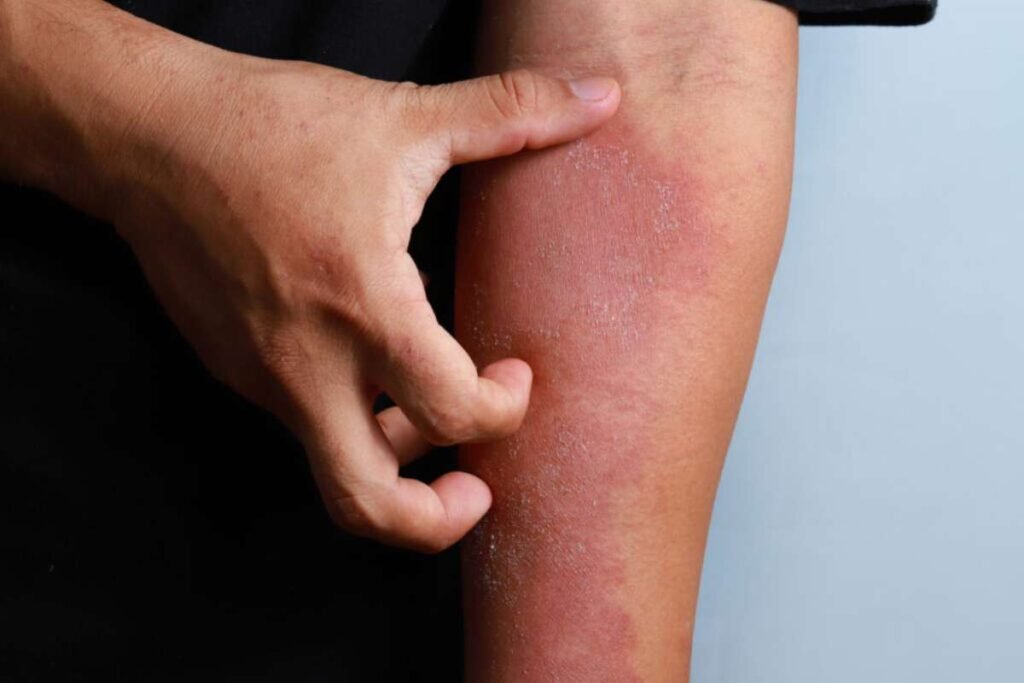
In a significant advancement for eczema treatment, Apogee Therapeutics has unveiled Phase 2 trial results for its lead candidate, APG777 — a novel IL-13-targeting antibody that may redefine the therapeutic landscape for moderate-to-severe atopic dermatitis. The company claims these results could challenge established therapies, particularly Sanofi and Regeneron’s market-leading Dupixent.
In the trial, 93% of patients receiving the higher dose of APG777 achieved a 75% improvement in the Eczema Area and Severity Index (EASI-75) by week 16. Even more striking, 62% reached EASI-90 (nearly clear skin), and 41% achieved full clearance (EASI-100). According to Fierce Biotech, these figures significantly outperform existing biologics and set a high bar for the industry.
CEO Michael Henderson emphasized the drug’s dual advantages: higher efficacy and monthly dosing. Unlike current standards like Dupixent, which requires biweekly injections, APG777 is administered just once a month, offering both clinical and lifestyle benefits for patients.
Importantly, the safety profile was also encouraging. No serious adverse events were reported, and injection site reactions were minimal. Apogee now plans to initiate a pivotal Phase 3 trial in 2025.
Market Confidence Soars as Apogee Stock Surges for Eczema Treatment
Following the announcement, Apogee Therapeutics saw a substantial spike in its stock price, reflecting investor enthusiasm over the drug’s potential. Analysts believe APG777 could become a category leader if the efficacy and safety data hold up in late-stage trials.
As reported by Investor’s Business Daily, APG777’s performance could pose a serious challenge to Dupixent, a biologic with over $8 billion in annual sales. With its once-monthly dosing and superior clearance rates, Apogee is being viewed as a potential disrupter in a market ripe for innovation.
Some market experts predict that if approved, APG777 could capture a large slice of the atopic dermatitis market, especially among patients seeking more convenient, less frequent dosing without compromising results.
Nektar Therapeutics Enters the Ring With REZPEG
While Apogee garners industry attention, Nektar Therapeutics is also pushing forward with its IL-2 pathway drug, rezpegaldesleukin (REZPEG). In its Phase 2b study, REZPEG demonstrated statistically significant reductions in eczema symptoms, with a strong tolerability profile. However, the efficacy data, though promising, appear more modest when compared to Apogee’s headline-grabbing results.
According to Insider Monkey, Nektar is now evaluating potential partners and preparing for Phase 3 development. Though REZPEG operates via a different mechanism, both companies share the ambition of transforming how chronic eczema is managed.
As these therapies move toward late-stage trials, the competition in eczema treatment is heating up. The next few years could see a major shift in patient care, driven by innovation, convenience, and improved outcomes.
Sources:
Also Read :- Healthcare 360 Magazine