[Source-Business Standard]
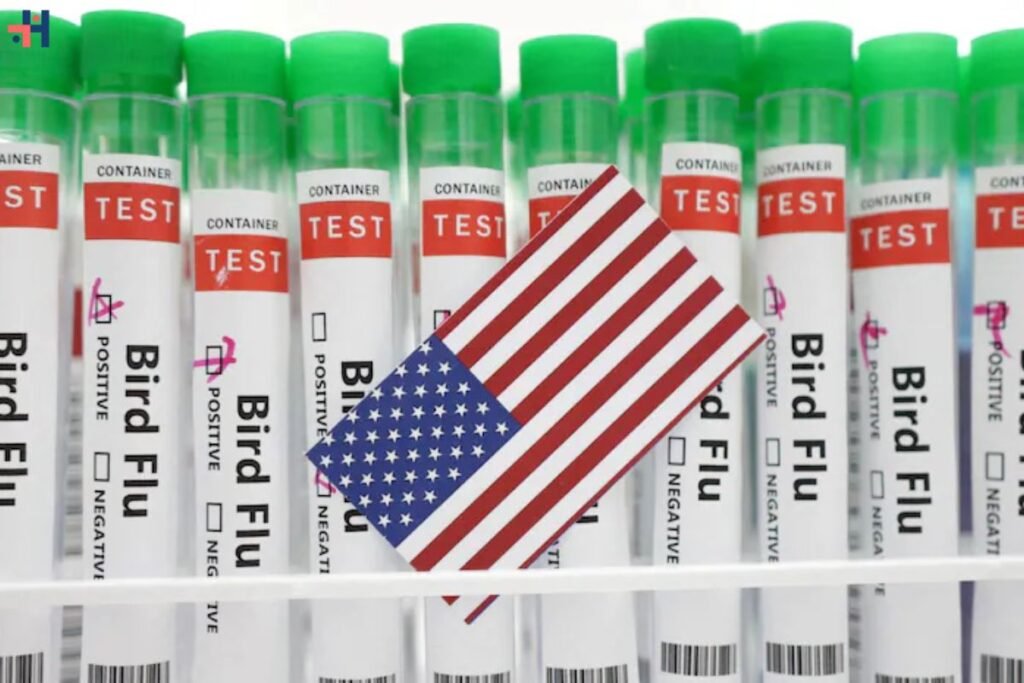
According to a report by the Financial Times on Thursday, the United States government is on the verge of finalizing an agreement to provide financial support for a late-stage trial of Moderna’s bird flu vaccine. The urgency comes as the outbreak continues to spread among dairy cows. Although Moderna did not explicitly confirm the funding, it acknowledged being engaged in discussions with the government regarding the advancement of its vaccine candidate.
Federal funding, expected to be channeled through the Biomedical Advanced Research and Development Authority (BARDA), is anticipated as early as next month. Sources close to the negotiations revealed that the funding package might include a commitment to procure doses should the late-stage trials prove successful. Despite requests for comments, the U.S. Department of Health and Human Services (HHS) has yet to respond to inquiries from Reuters.
Moderna’s Bird Flu Vaccine Development and Market Response
Moderna, a frontrunner in the development of mRNA-based vaccines, is currently evaluating its experimental shot, mRNA-1018, against various strains of the bird flu virus, including the prevalent H5N1 variant. The company has recently concluded dosing in an early-to-mid stage study of the vaccine and is awaiting data analysis.
The news of potential government funding has had a positive impact on Moderna’s stock performance, with shares surging approximately 40% since April 1, coinciding with the second documented human case of bird flu in the United States. Pre-market trading on Thursday saw a further increase of about 3%, with Moderna’s stock reaching $151.25. Despite the availability of Moderna’s bird flu vaccines and antivirals in the U.S. stockpile, officials recognize the need for expanded reserves in anticipation of major epidemics or pandemics.
U.S. nears deal to fund Moderna’s bird flu vaccine trial
Government Preparedness and Collaborative Efforts
In addition to funding Moderna’s vaccine trial, the U.S. government has been proactive in bolstering its defenses against the bird flu outbreak. Current efforts include manufacturing approximately 4.8 million doses of CSL Seqirus’ Moderna’s bird flu vaccine. Furthermore, discussions with pharmaceutical giants Pfizer and Moderna are underway to explore the feasibility of an mRNA-based vaccine. Last week, authorities confirmed yet another human infection, underscoring the persistent threat posed by the virus since its initial detection in dairy cattle in late March. These collaborative endeavors highlight a concerted effort to mitigate the spread of bird flu and safeguard public health.
By closely monitoring developments in Moderna’s bird flu vaccine research and fostering partnerships with leading biotech firms, the U.S. government aims to fortify its preparedness against emerging infectious diseases, ensuring a swift and effective response to future outbreaks.