[Source-CNBC]
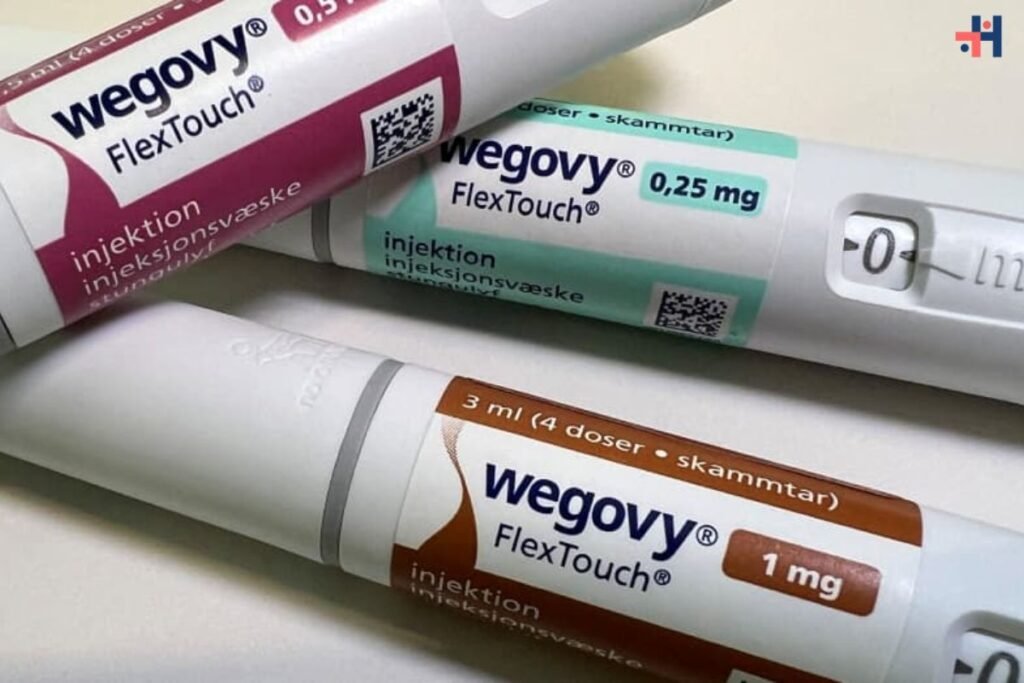
In a significant development, the renowned weight loss medication Wegovy has received approval from the Food and Drug Administration (FDA) for a novel application: reducing the risk of heart attacks, strokes, and cardiovascular-related mortality among adults with heart disease who are overweight or obese. This expansion of Wegovy’s indication signals a pivotal moment in the realm of cardiovascular care and could potentially enhance accessibility for a broader spectrum of patients, potentially leading to expanded insurance coverage. Novo Nordisk, the pharmaceutical giant behind Wegovy, has also initiated steps to secure an expanded label in the European Union, heralding a global shift in the drug’s therapeutic landscape.
Clinical Validation and Regulatory Approval
The FDA’s decision to broaden it’s usage stemmed from compelling findings derived from a comprehensive study involving over 17,000 adults aged 45 and above. Participants were administered either Wegovy injections or a placebo and were monitored over several years. The results revealed a notable disparity in cardiovascular outcomes, with a lower incidence of heart attacks, strokes, and cardiovascular fatalities observed among those who received Wegovy compared to the placebo group. While the precise mechanisms underlying it’s cardiovascular benefits remain subject to further investigation, experts underscored the transformative impact of prioritizing obesity management in individuals with a heightened risk of cardiovascular complications.
Economic Implications and Access Challenges
Despite Wegovy’s efficacy, concerns regarding its affordability and long-term sustainability persist. With a list price exceeding $1,300 per month’s supply, and the likelihood of prolonged therapy durations, cost considerations have prompted some employers and health plans to reconsider coverage policies, leading to access restrictions and budgetary constraints. The updated label for Wegovy is anticipated to intensify pressure on payers and employers to reassess coverage policies, particularly given the substantial proportion of eligible patients who may rely on Medicare for healthcare coverage.
Wegovy wins FDA approval for heart health
Supply Shortages and Market Dynamics
Additionally, the pharmaceutical industry faces logistical hurdles in meeting escalating demand for drugs like Wegovy. Presently, shortages plague nearly all available doses of Wegovy, exacerbating concerns surrounding medication accessibility. While Novo Nordisk endeavors to augment supply volumes, challenges persist in ensuring equitable distribution channels and mitigating supply chain disruptions.
Despite Wegovy’s pronounced cardiovascular benefits, clinicians remain vigilant regarding potential adverse effects, particularly among older adults with a history of heart disease. Concerns regarding muscle mass loss and frailty underscore the imperative of individualized risk assessment and close monitoring in high-risk populations. Moreover, the prevalence of off-label use for cosmetic purposes underscores the need for comprehensive patient education and responsible prescribing practices.
Future Outlook and Patient-Centric Care
As the medical community navigates the evolving landscape of cardiovascular risk management, It’s expanded indication heralds a new era of personalized and preventive healthcare. With millions of eligible individuals poised to benefit from It’s cardiovascular protection, concerted efforts are warranted to address access disparities, optimize therapeutic outcomes, and uphold patient-centered care principles. In doing so, stakeholders can collectively strive to realize the transformative potential of Wegovy in combating cardiovascular disease and improving public health outcomes.